Waiver of Documentation of Consent
46.117(c): An IRB may waive the requirement for the investigator to obtain a signed consent form for some or all subjects if it finds any of the following to be true:
- That the only record linking the subject and the research would be the consent document and the principal risk would be potential harm resulting from a breach of confidentiality. Each subject will be asked whether the subject wants documentation linking the subject with the research, and the subject's wishes will govern; or
- That the research presents no more than minimal risk of harm to subjects and involves no procedures for which written consent is normally required outside of the research context; or
- If the subjects or legally authorized representatives are members of a distinct cultural group or community in which signing forms is not the norm, that the research presents no more than minimal risk of harm to subjects and provided there is an appropriate alternative mechanism for documenting that informed consent was obtained.
Note: Options 1 and 2 are equivalent to FDA's #56.109(c)(1)
Frequently called Verbal Consent, the process is more correctly referred to as Consent with Waiver of Documentation. The investigator must obtain consent following the same requirements as written consent but the subject does not sign a consent document.
Waiver of documentation of consent is permitted only under these limited set of circumstances. To waive documentation of consent, the research must meet the regulatory criteria of 45 CFR 46.117(c) or 21 CFR 50.109(c).
Obtaining Verbal Consent
The consent process still needs to adhere to all the requirements of consent. If the study is subject to HIPAA, written HIPAA authorization may still be required unless the study also qualifies for alteration of the requirement for written HIPAA Authorization. IRB provides a template for this type of consent, with no signature line
Examples of situations and options for verbal consent:
- Consent form using the Emory consent template without a page for subject or investigator signatures. This option would apply when the basis of waiver was 45 CFR 46.117(c)(1) where the consent form would have been the only link to the research. It could also apply when the plan is to document the consent process place in the research or medical records. This latter situation is risky because it may be difficult to prove during an audit that consent took place.;
- Consent form using the Emory template but substituting the usual signature page with the IRB's documentation of verbal consent page. When assent must be obtained, consent form should also include the page for documentation of verbal assent. A typical scenario for this would be when consent, assent and HIPAA Authorization are obtained over the telephone.;
- Consent form using the Emory consent template with a page for documentation of verbal consent by the investigator and a stand-alone HIPAA authorization. Some studies will qualify for waiver of documentation of consent but still not qualify for verbal HIPAA authorization. For example, the IRB could waive the requirement for written consent for subjects seen in clinic but the study wouldn't meet the criteria for alteration of HIPAA because it would be practicable to obtain written HIPAA .
- Two consent forms, one for written consent and HIPAA Authorization and one for verbal consent. In many studies, some subjects will be seen in-person in clinic and others will be consented over the telephone. The consent of subjects who are seen in clinic include written consent and HIPAA authorization (or verbal consent + written HIPAA Authorization) and the consent of subjects who are enrolled via telephone, would be verbal.
Special Case: Consent process when the invitation is by mail or email
This option most often applies to surveys and questionnaires but can also include a specimen collection kit. The questionnaire or specimen collection kit should be accompanied by a letter, webpage or consent form inviting the individual to participate. The letter, webpage instructions or consent form should include:
- The required elements of consent; and
- A statement that completion and return of the questionnaire, web survey, specimen kit, etc. indicates willingness to participate.
- May also provide a checkbox for the person to indicate that they have read the information and agree to participate
This process is sometimes referred to as implied consent, which is not a term recognized by OHRP. They would consider this to be informed consent with waiver of documentation (provided the IRB has waived documentation under 45 CFR 46.117(c)(1) or (2)). See OHRP's website for a discussion about implied consent.
Documenting Verbal Consent
The IRB may approve a waiver or alteration of HIPAA provided that the research meets the criteria outlined in 45 CFR 164.512(i.)(2)(ii).
Depending on the circumstances, it may still be appropriate to document that the consent process took place.
Waiver under 45CFR46.117(c)(1)
When a waiver is issued under 45CFR46.117(c)(1) consent should only be documented when the subject requests to be linked to the research. A consent form should be available when using a waiver of documentation under (c)(1) in case the subject wishes to be linked. The form of documentation could include any of the following:
- Note written in the study subject's record
- On a consent/assent documentation form with a signature page
- On a consent form with a page for documentation of verbal consent and when applicable, assent and HIPAA Authorization
Waiver under 45CFR46.117(c)(2)
When a waiver is issued under 45CFR46.117(c)(2) consent documentation could include any of the following:
- No documentation (strongly discouraged)
- Note written in the study subject's research or medical record
- On a form created specifically for documentation of verbal consent and when applicable, assent and HIPAA Authorization
- On a consent form with a page for documentation of verbal consent and when applicable, assent and HIPAA Authorization
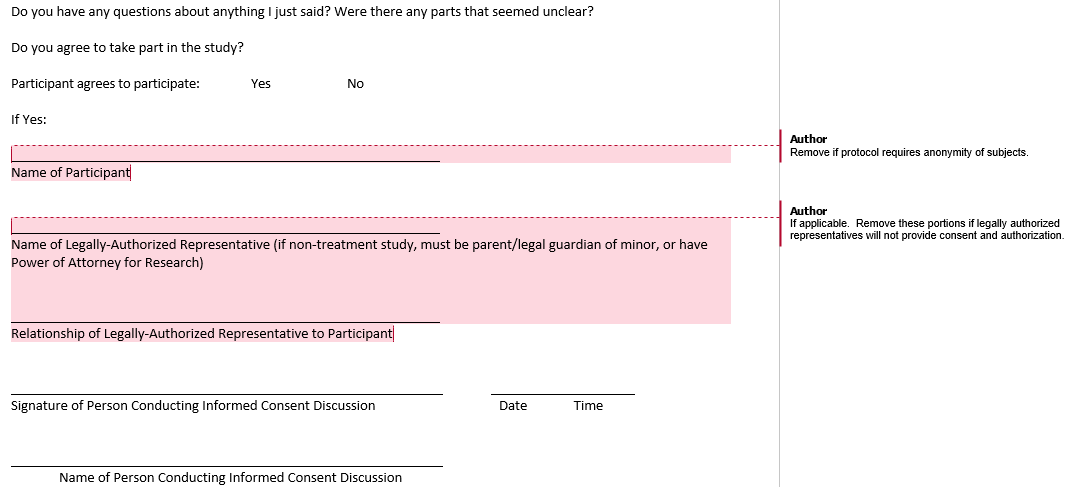
Example Signature Page to Document Consent Process:
When can the IRB waive the requirement for written consent for audiotaping for patient-subjects?
If the subject is physically present and is a patient at Emory, the IRB can not waive the requirement. This effectively eliminates waivers for videotaping of patient-subjects. If the research involves a telephone interview, then the IRB will consider a request for a waiver.
The investigator must do the following:
- Inform the prospective subject that they would like to audiotape the conversation (to comply with state wiretapping laws);
- If the prospective participant agrees, the consent conversation, including the required information about audiotaping must be recorded;
- If the subject consents, the audiotape of the consent conversation must be retained for 6 years (to meet HIPAA and hospital policy).
Waiver of Documentation of HIPAA Authorization
45 CFR 164.164.512:
Uses and disclosures for which an authorization or opportunity to agree or object is not required.
When the research qualifies for a Waiver or Alteration of HIPAA under 45 CFR 164.512(i)(2)(ii) (PDF) then "A covered entity may use or disclose protected health information without the written authorization of the individual, as described in §164.508, or the opportunity for the individual to agree or object as described in 164.510, in the situations covered by this section, subject to the applicable requirements of this section. When the covered entity is required by this section to inform the individual of, or when the individual may agree to, a use or disclosure permitted by this section, the covered entity's information and the individual's agreement may be given orally."


